45 open-label extension study
› ct2 › showAn Extension Study to Evaluate the Long-term Safety and ... Feb 25, 2021 · This is a phase 3, open-label, multicenter, extension study to evaluate the long-term safety and efficacy of pegcetacoplan (APL-2) in subjects with geographic atrophy (GA) secondary to age-related macular degeneration (AMD) who participated in Study APL2-103 (NCT03777332) or completed the treatment at Month 24 of either Study APL2-303 (Derby ... UEG 2022 - Programme Timezone of UEG Week 2022 16:57:11 CEST (UTC+2) Saturday, October 15, 2022. Programme ; Chairs & Speakers ; Favourites ; PDF Programme ; Login
clinicaltrials.gov › ct2 › showFourier Open-label Extension Study in Subjects With ... Mar 15, 2017 · This is a multicenter, open-label extension (OLE) study designed to assess the extended long-term safety of evolocumab in subjects who have completed the FOURIER trial (Study 20110118). FOURIER is a randomized placebo-controlled study of evolocumab, in patients with clinically evident atherosclerotic CVD on stable effective statin therapy.

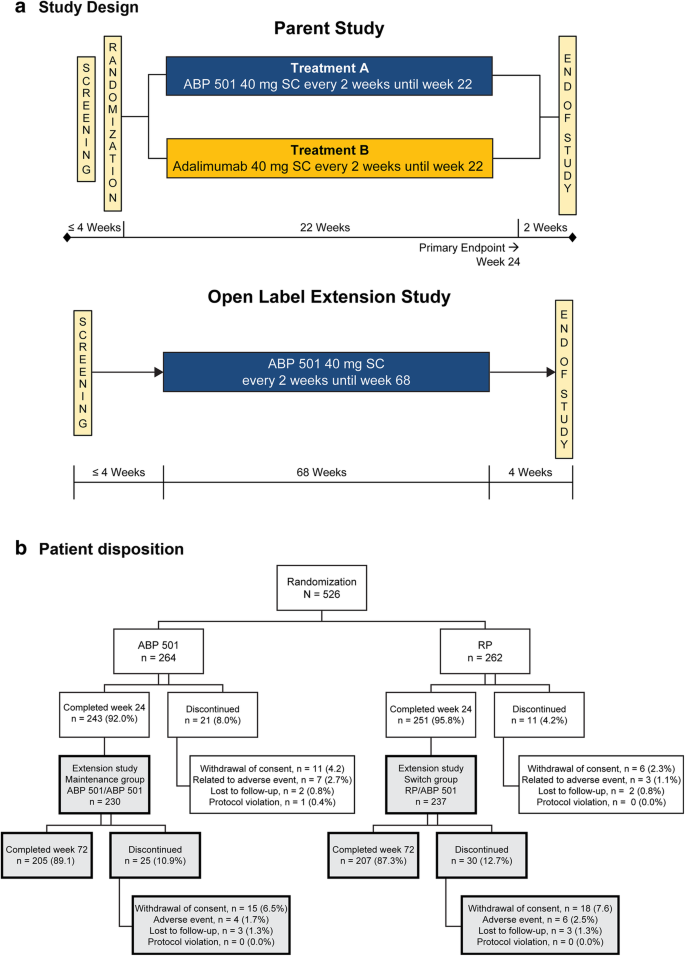
Open-label extension study
pubmed.ncbi.nlm.nih.gov › 34597534Long-term safety and efficacy of dupilumab in patients with ... Methods: TRAVERSE was an open-label extension study in 362 hospitals and clinical centres across 27 countries that assessed the safety and efficacy of dupilumab 300 mg every 2 weeks up to 96 weeks in adults and adolescents (aged 12-84 years) with moderate-to-severe or oral-corticosteroid-dependent severe asthma who had completed a previous ... › article › releasesCapricor Therapeutics to Present One-Year Results from HOPE-2 ... Oct 06, 2022 · Capricor Therapeutics (NASDAQ: CAPR) a biotechnology company focused on the development of transformative cell and exosome-based therapeutics for the treatment and prevention of muscular and other select diseases, announced today that the Company will present one-year safety and efficacy results from its HOPE-2 open-label extension study with ... › news › homeKalVista Pharmaceuticals Announces Initiation of KONFIDENT-S ... Aug 23, 2022 · Initiation of this OLE study follows submission to the FDA of pivotal toxicology studies intended to support the eventual NDA filing. For more information on the open-label extension study, please ...
Open-label extension study. clinicaltrials.gov › ct2 › showPhase 2 Study of Amcenestrant (SAR439859) Versus Physician's ... Aug 16, 2019 · The duration of the study for an individual participant will include a period to assess eligibility (screening period) of up to 4 weeks (28 days), a treatment period of at least 1 cycle (28 days of study treatment), and an end of treatment (EOT) visit at least 30 days (or until the participant receive another anticancer therapy, whichever is earlier) following the last administration of study ... en.wikipedia.org › wiki › Clinical_trialClinical trial - Wikipedia A clinical trial might also include an extended post-study follow-up period from months to years for people who have participated in the trial, a so-called "extension phase", which aims to identify long-term impact of the treatment. The biggest barrier to completing studies is the shortage of people who take part. Home Page: The Journal of Sexual Medicine - jsm.jsexmed.org Home Page: The Journal of Sexual Medicine - jsm.jsexmed.org › news › homeKalVista Pharmaceuticals Announces Initiation of KONFIDENT-S ... Aug 23, 2022 · Initiation of this OLE study follows submission to the FDA of pivotal toxicology studies intended to support the eventual NDA filing. For more information on the open-label extension study, please ...
› article › releasesCapricor Therapeutics to Present One-Year Results from HOPE-2 ... Oct 06, 2022 · Capricor Therapeutics (NASDAQ: CAPR) a biotechnology company focused on the development of transformative cell and exosome-based therapeutics for the treatment and prevention of muscular and other select diseases, announced today that the Company will present one-year safety and efficacy results from its HOPE-2 open-label extension study with ... pubmed.ncbi.nlm.nih.gov › 34597534Long-term safety and efficacy of dupilumab in patients with ... Methods: TRAVERSE was an open-label extension study in 362 hospitals and clinical centres across 27 countries that assessed the safety and efficacy of dupilumab 300 mg every 2 weeks up to 96 weeks in adults and adolescents (aged 12-84 years) with moderate-to-severe or oral-corticosteroid-dependent severe asthma who had completed a previous ...

































02467-6.fp.png)


Komentar
Posting Komentar