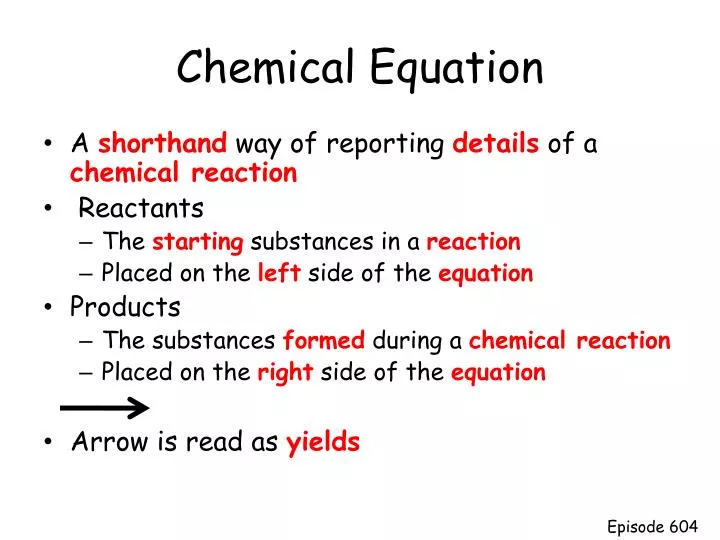
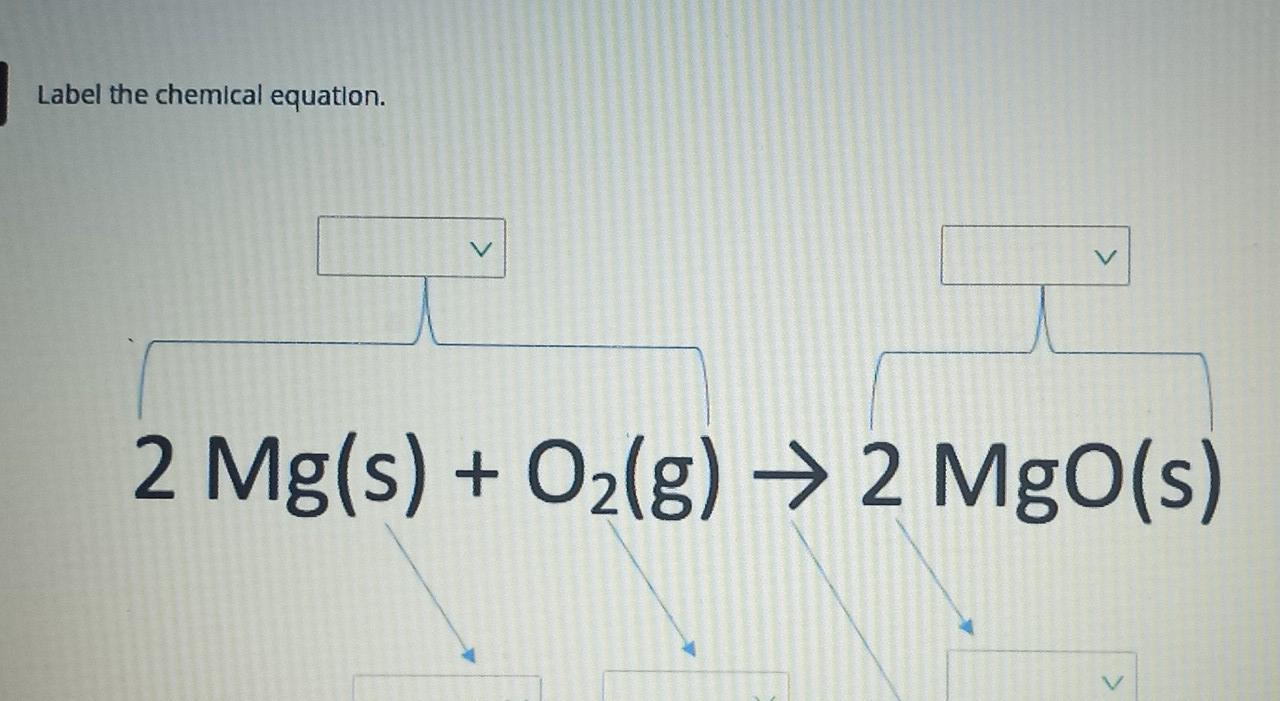
42 chemical equation with labels
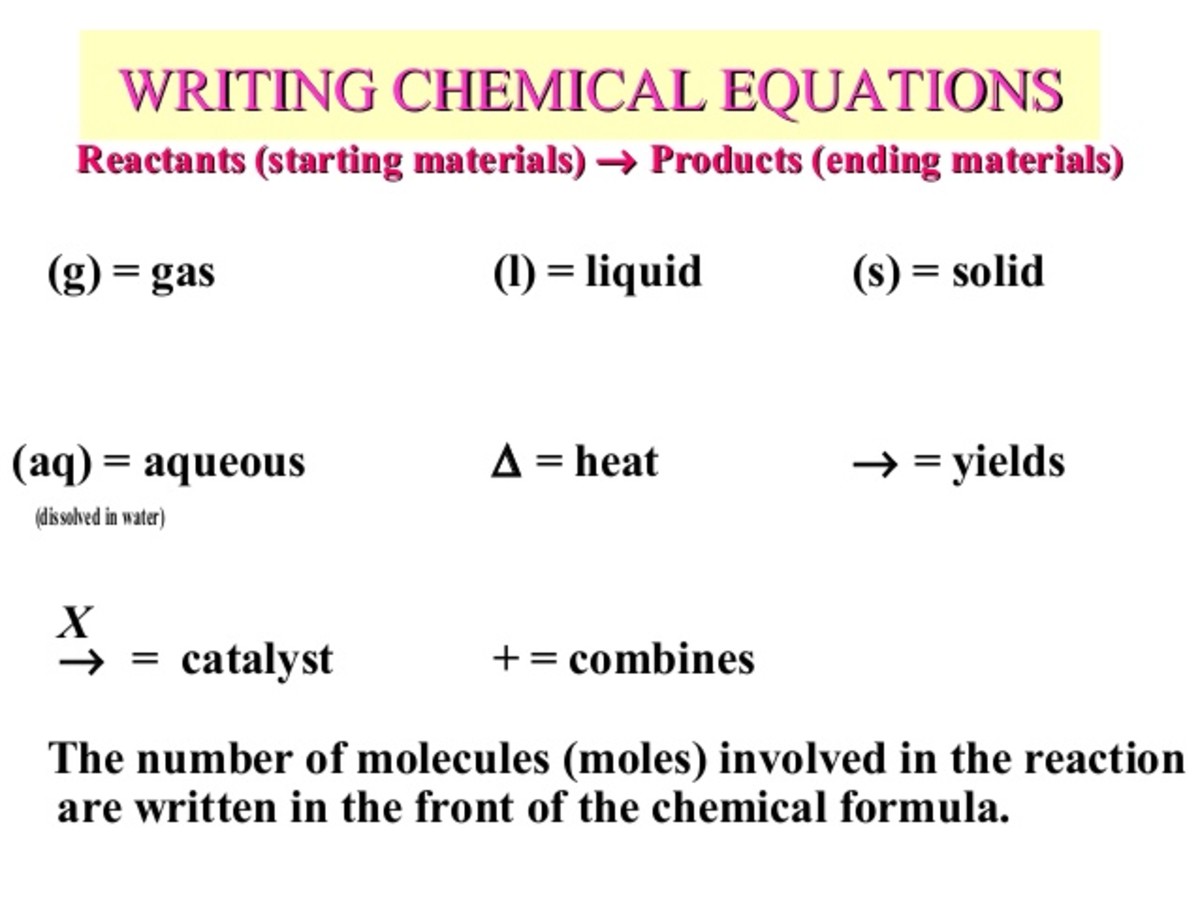
How would you label each formula in the chemical equation below ... Jan 23, 2017 ... We designate the reactants as written........ The reactants are on the LEFT HAND SIDE of the equation. The products are on the right hand ... Chapter 4 Quantities of reactants and products Phase labels: letters written in parenthesis after a reactant or product to indicate whether the substance is a solid (s), liquid (l), gas (g) or dissolved in ...
What are Chemical Equations? Detailed Explanation, Examples -... Chemical Equation: CaCl 2 + 2AgNO 3 → Ca(NO 3) 2 + 2AgCl↓. Ionic Equation: Ca 2+ + 2Cl – + 2Ag + + 2NO 3 – → Ca 2+ + 2NO 3 – + 2AgCl↓. Comparing the reactants and the products of the ionic equation and the chemical equation, it can be observed that the Ca 2+ (calcium ion) and the NO 3 – (nitrate) ions are present on both sides of the ionic equation. These ions are referred to as spectator ions because they do not participate in the chemical reaction.

Chemical equation with labels
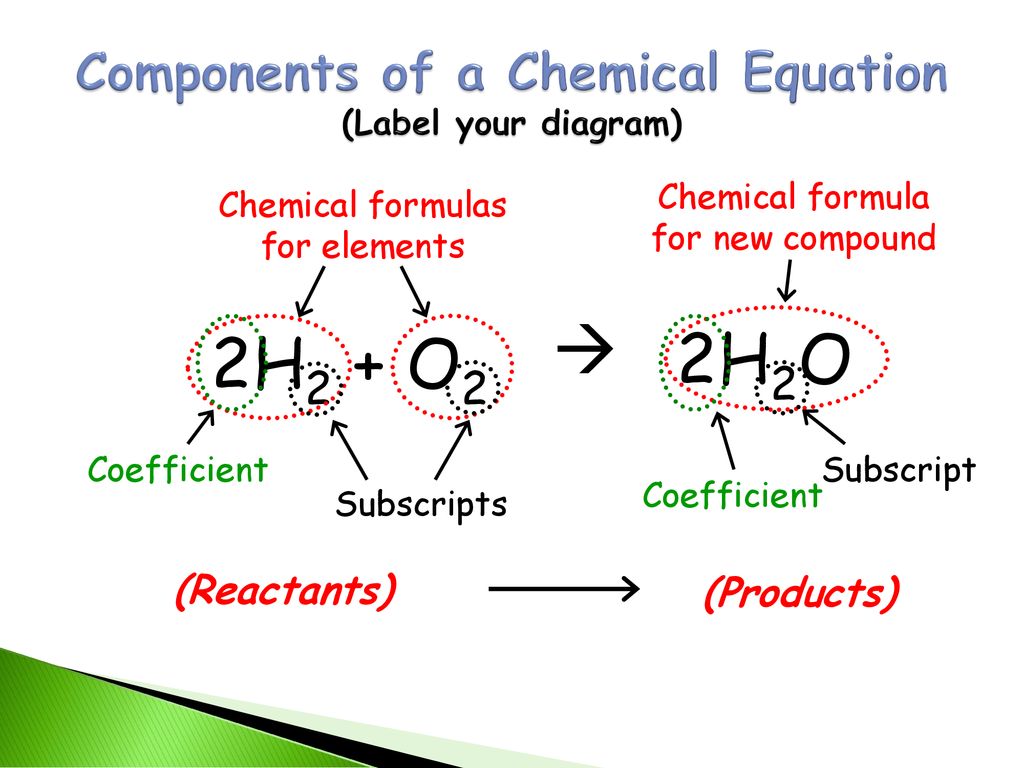
3.10: Writing and Balancing Chemical Equations Jul 1, 2019 · A chemical reaction is described by a chemical equation, an expression that gives the identities and quantities of the substances involved in a reaction. A chemical equation shows the starting compound(s)—the reactants—on the left and the final compound(s)—the products—on the right, separated by an arrow. In a balanced chemical equation, the numbers of atoms of each element and the total charge are the same on both sides of the equation. 7.3: The Chemical Equation - Chemistry LibreTexts Jul 18, 2022 ... Reactants and Products ... To describe a chemical reaction, we need to indicate what substances are present at the beginning and what substances ... The Chemical Equation, Its Parts, Labels and State Symbols Jan 8, 2013 ... The chemical equation is a shorthand way of describing a chemical reaction. A chemical equation may consist of simple formulas or very complex, ...
Chemical equation with labels. Chemical Nomenclature and Chemical Formulas - Owlcation Jan 10, 2015 · Symbols are used as shorthand abbreviations for elements composed of a single capital letter or a capital letter and one or two small letters like H, O, Cl, Na, or Unq. Formulas are combinations of symbols such as CO 2 for carbon dioxide, C 6 H 22 O 11 for table sugar and HCl for hydrochloric acid. Chemical reactions are composed of elements and compounds which are described through chemical equations using symbols and formulas. Labeling A Chemical Equation Part 2 - YouTube Aug 5, 2012 ... The instructions for labeling a chemical equation. Label each reactant and product in the given chemical reaction. Indicate the name of the starting reactant and the final product. ... Determine the product of the given reaction. ... Write the products for the given sequence of ... The Chemical Equation – Introductory Chemistry – 1st Canadian... Many chemical equations also include phase labels for the substances: (s) for solid, (ℓ) for liquid, (g) for gas, and (aq) for aqueous (i.e., dissolved in water). Special conditions, such as temperature, may also be listed above the arrow. For example: 2NaHCO 3 (s) → 200°C Na 2 CO 3 (s) + CO 2 (g) + H 2 O (ℓ) Key Takeaways
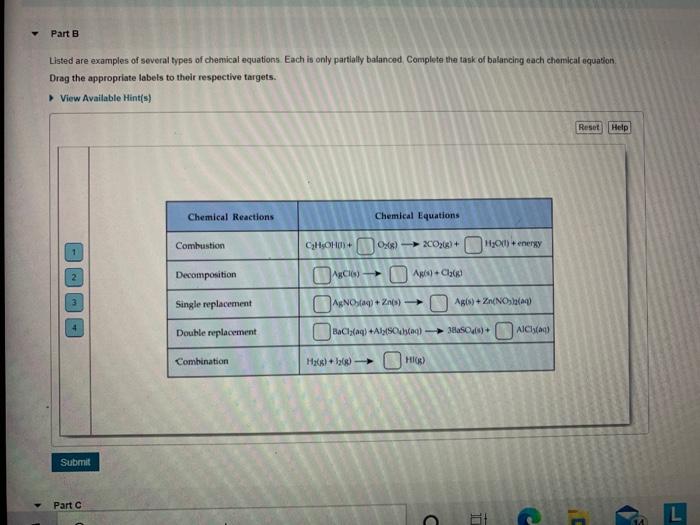
3.1: Chemical Equations - Chemistry LibreTexts Aug 17, 2022 ... A chemical reaction is described by a chemical equation, an expression that gives the identities and quantities of the substances involved in a ... Laebling A Chemical Equation Part I - YouTube Aug 5, 2012 ... Labeling chemical equations and identifing reactants, yield and products. How to Write a Chemical Equation (with Pictures) - wikiHow Oct 7, 2022 · The general equation takes the form of AB + CD → AD + CB, where A and C are cations and B and D are anions. You also want to determine the charges of each ion. For example: AgNO 3 + NaCl → ? The cations are Ag +1 and Na+1. The anions are NO31- and Cl1-. 2 Switch the ions to build the products. Visually understanding balancing chemical equations 7 years ago. If the number is before the molecule, then you have two of those molecules, e.g. 2NaCl means two molecules of NaCl. If the number is in subscript (small, bottom right) after an element, then that element is repeated twice, e.g. H₂O (water) means that the molecule has two hydrogen atoms and one oxygen atom.
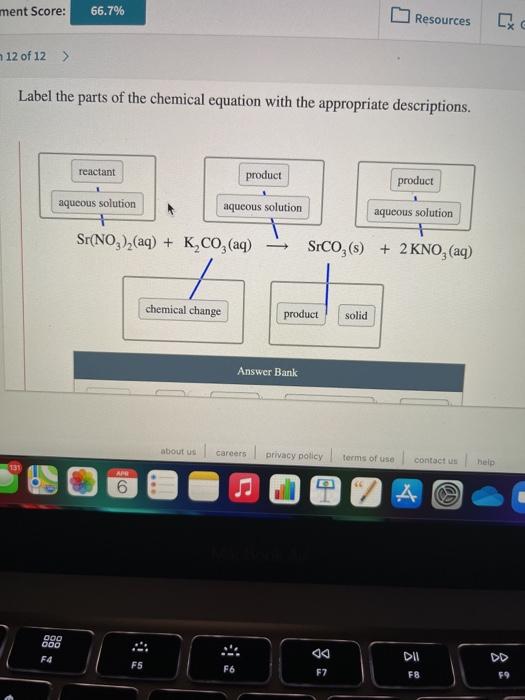
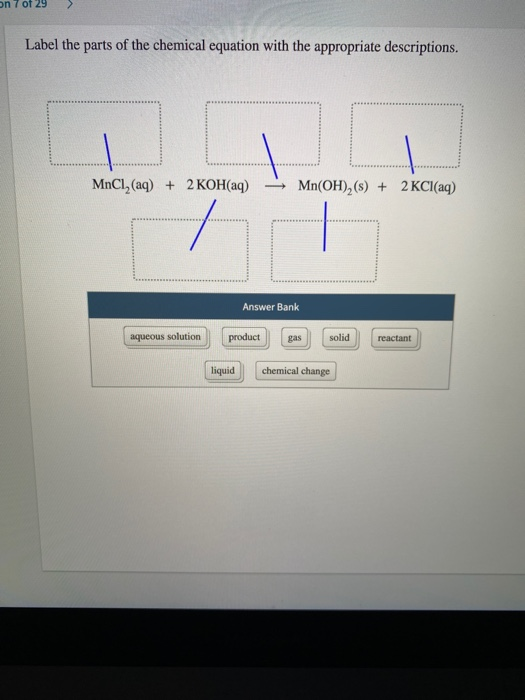
Align and label in chemical equation - TeX - LaTeX Stack Exchange Apr 17, 2017 ... Align and label in chemical equation · How to only label the second, third and sixth line with 1, 2, 3? · the arrow with catalyst in first line is ... 3.10: Writing and Balancing Chemical Equations Jun 24, 2021 · A chemical reaction is described by a chemical equation, an expression that gives the identities and quantities of the substances involved in a reaction. A chemical equation shows the starting compound(s)—the reactants—on the left and the final compound(s)—the products—on the right, separated by an arrow. In a balanced chemical equation, the numbers of atoms of each element and the total charge are the same on both sides of the equation. Label the parts of the chemical equation with the appropriate ... Nov 14, 2020 ... Label the parts of the chemical equation with the appropriate descriptions. Sr(NO3)2(aq) + K2CO3(aq) ⟶ SrCO3(s) + 2KNO3(aq). answer bank-. The Chemical Equation, Its Parts, Labels and State Symbols Jan 8, 2013 ... The chemical equation is a shorthand way of describing a chemical reaction. A chemical equation may consist of simple formulas or very complex, ...
7.3: The Chemical Equation - Chemistry LibreTexts Jul 18, 2022 ... Reactants and Products ... To describe a chemical reaction, we need to indicate what substances are present at the beginning and what substances ...
3.10: Writing and Balancing Chemical Equations Jul 1, 2019 · A chemical reaction is described by a chemical equation, an expression that gives the identities and quantities of the substances involved in a reaction. A chemical equation shows the starting compound(s)—the reactants—on the left and the final compound(s)—the products—on the right, separated by an arrow. In a balanced chemical equation, the numbers of atoms of each element and the total charge are the same on both sides of the equation.





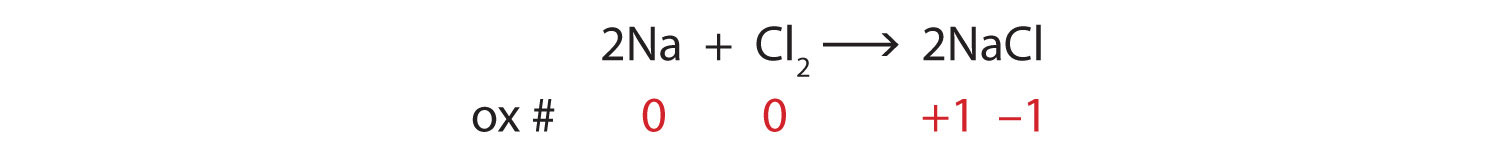
















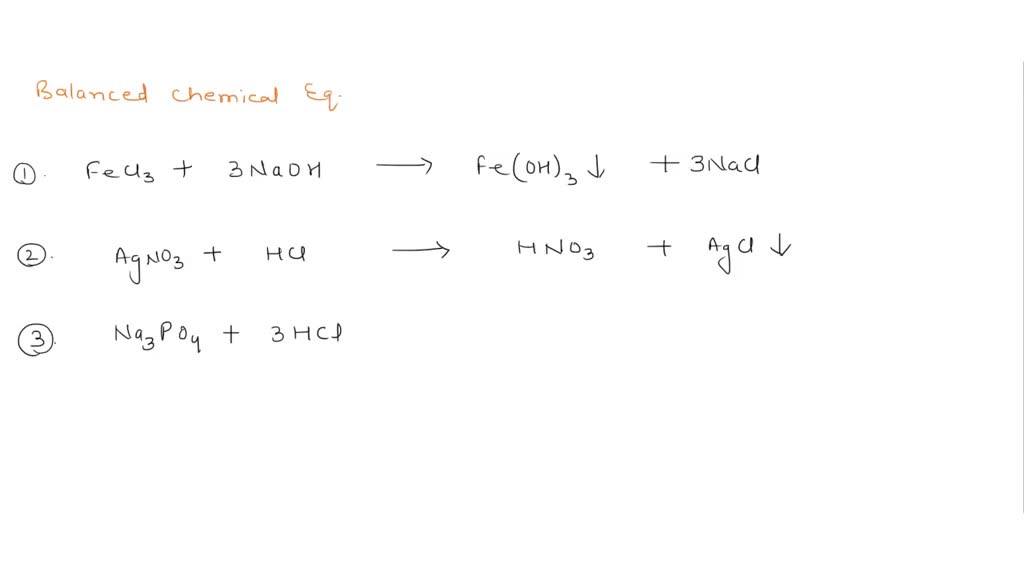



Komentar
Posting Komentar